Abstract
Introduction Anti-CD19 chimeric antigen receptor (CAR) T-cells therapy has achieved unprecedented efficacy in refractory or relapsed (R/R) B-cell acute lymphoblastic leukemia (B-ALL) patients, with up to a 90% complete remission (CR) rate. However, a large body of clinical data has shown that the relapse rate post CD19 CAR-T therapy remains high. Our published study of 254 patients with R/R B-ALL treated with CD19 CAR-T showed the median time to relapse following CAR-T cells infusion was 100 days. Patients who received consolidative allogeneic hematopoietic stem cell transplantation (allo-HSCT) post CAR-T therapy demonstrated much higher overall survival (OS) and leukemia-free survival (LFS) compared to those who received CAR-T therapy alone (1-year OS of 77.8% vs. 35.4%, p<0.0001; 1-year LFS of 70.9% vs. 33.3%, p<0.0001, respectively). However, there are still many patients who cannot undergo allo-HSCT, including those who have relapsed following allo-HSCT and elderly patients. Prolonging disease-free survival (DFS) after CAR-T therapy in these patients remains a challenge for physicians. Studies have shown that the histone deacetylase (HDAC) inhibitor chidamide can up-regulate T-cell activity and down-regulate derived suppressor cell (MDSC) expression. Chidamide also promotes the expression of CD22 on the surface of B-cell tumor cells in vitro and in vivo, and enhances CAR-T function. Here we explore the efficacy of chidamide as maintenance therapy post CAR-T in B-ALL.
Patients and methods We analyzed 19 patients with R/R B-ALL who received CD19 CAR-T at our center (NCT04546893) between October 2019 and July 2022. All patients achieved CR on day 30 following CD19 CAR-T cells infusion and were unable to bridge to allo-HSCT within 3 months. We administered chidamide as maintenance therapy to all 19 patients between 30 to 90 days after CAR-T infusion. Chidamide was continuously administered until the patient relapsed, transferred to transplant, or had intolerable side effects.
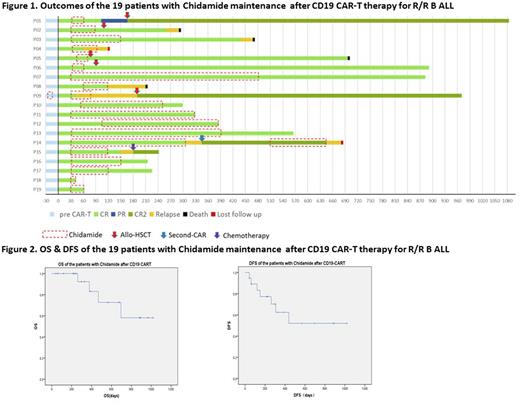
Results On Day 30 post CD19 CAR-T, 19/19 (100%) patients achieved CR or CR with incomplete count recovery (CRi)(Figure 1). Sixteen of 18 (88.89%) who had bone marrow involvement achieved minimal residual disease (MRD)-negative CR. The median time from CAR-T cell infusion to start chidamide therapy was 30 days (range: 30-90 days) and the median duration of chidamide administration was 90 days (range: 12-420 days). No patient experienced cytokine release syndrome (CRS) or encephalopathy while taking chidamide. All side effects that occurred were mild. Two patients experienced nausea and vomiting and one patient had mild bone marrow suppression. No other side effects occurred. The median follow-up time was 12 months (range: 1-33 months), the 12-month OS rate was 83.1% and the 12-month DFS rate was 62.4% (Figure 2). Five patients received allo-HSCT following chidamide maintenance and 13 patients who did not undergo transplantation had 12-month OS of 83.3%, 12-month DFS of 62.9%. Six patients were monitored for the proportion of CAR-T cells in T lymphocytes following chidamide therapy. In all six patients, the proportion of CAR-T cells increased but to different degrees.
Conclusions For those patients with R/R B-ALL for whom allo-HSCT is not available following CD19 CAR-T therapy, HDAC inhibitor chidamide as maintenance therapy is a viable option to reduce the risk of relapse rate following CD19 CAR-T therapy. This study demonstrated that chidamide could prolong OS and DFS in some patients who achieved CR after CD19 CAR-T therapy. Chidamide was safe, inexpensive and convenient to take orally. The inclusion of more patients and extended observation time is needed for further evaluation of chidamide maintenance therapy following CAR-T cell therapy in patients with R/R B-ALL.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal